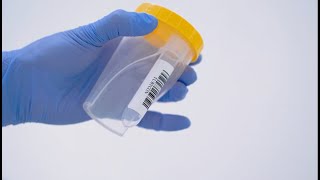
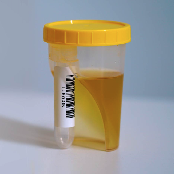
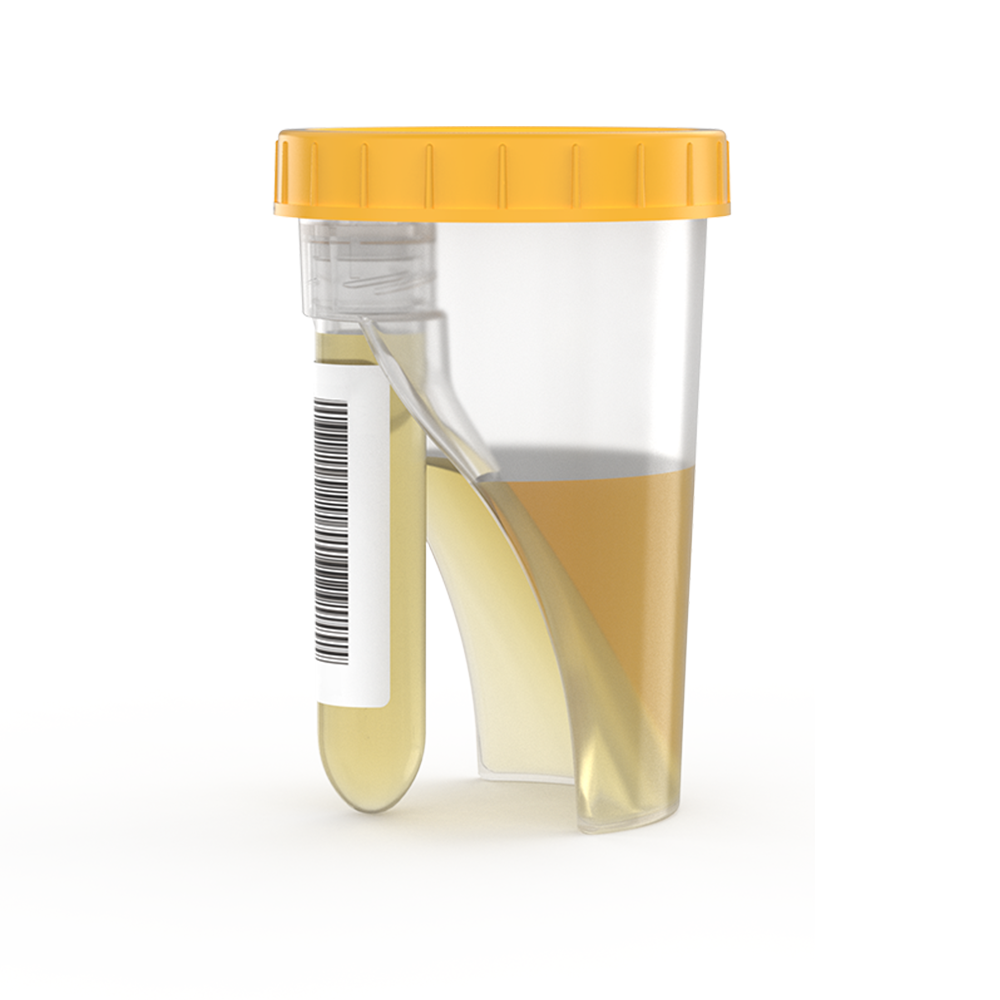
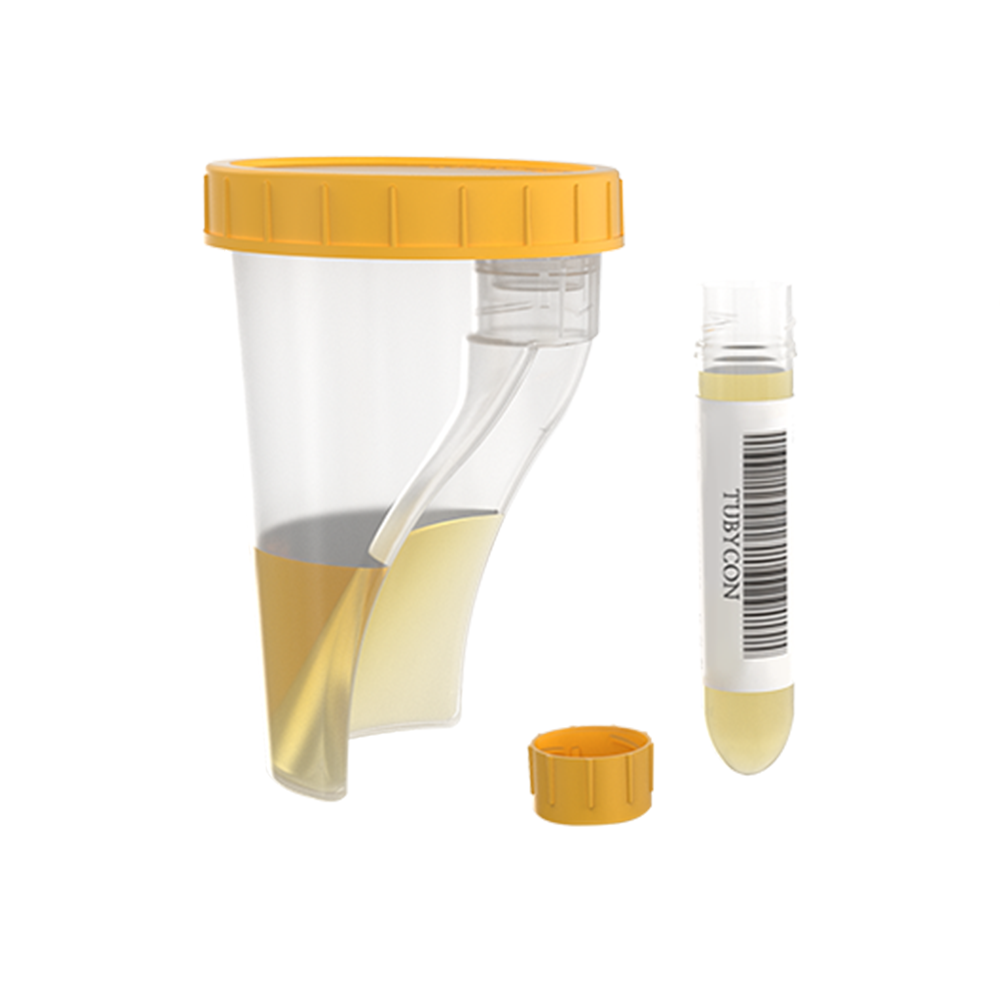
TUBYCON Single Tube Urine Test Cup Kit
-
Payment
P@yGOS(PayPal) , T/T
-
MOQ
6,000 ea
-
Supply Ability
9,999,999 ea per One-Time
-
Supply Details
Customization
Negotiable
-
Country of sale
Americas, Europe, Asia, Middle East, World Wide
-
PRICE
-
EXW
USD 0.40
(1 ea)
-
ITEM SPECIFICS
-
Brand
Model TUBYCON-U_01TUBYCON
-
origin
Republic of Korea
-
Size(Capacity)
90mL
-
Function
Infection prevention, specimen identification accuracy, improved workflow
-
Material
Polypropylene (PP), Polyethylene (PE)
-
Color
Transparent (cup, tube), Yellow (cup cap)
-
Dimensions
Not applicable
-
Weight
26.2g
-
Style
Integrated cup with a detachable tube
-
Expiry Date
5 years
-
Condition
New, Single-use, Non-sterile
-
Features
Integrated cup and tube design, detachable tube, compatible with lab automation
-
Gender
Not applicable
-
age-appropriate
For use by healthcare professionals only
PRODUCT DESCRIPTION
TUBYCON-U Urine Sample Collection Single Kit – Detailed Description
Key Features
· Purpose
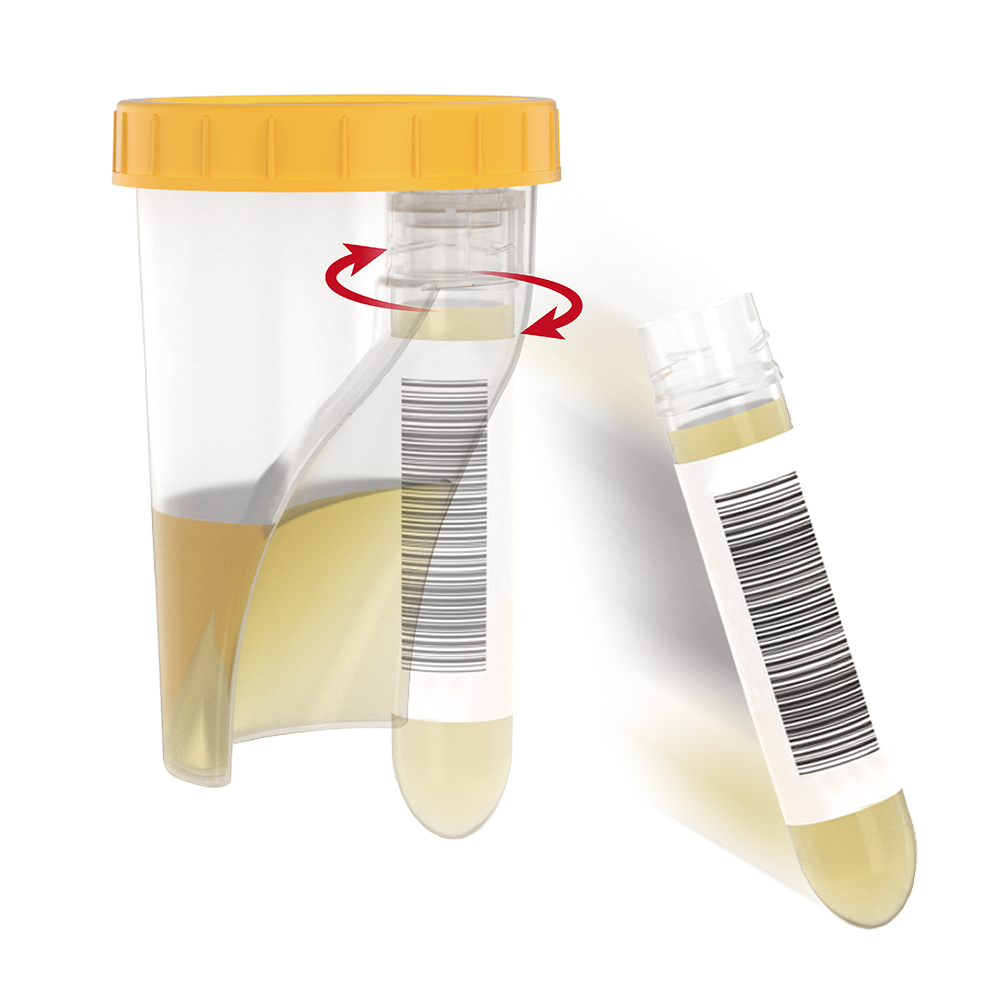
This is an integrated urine collection kit designed for hygienic and efficient collection and handling of urine samples in clinical laboratories and hospital settings.
· Effects/Benefits
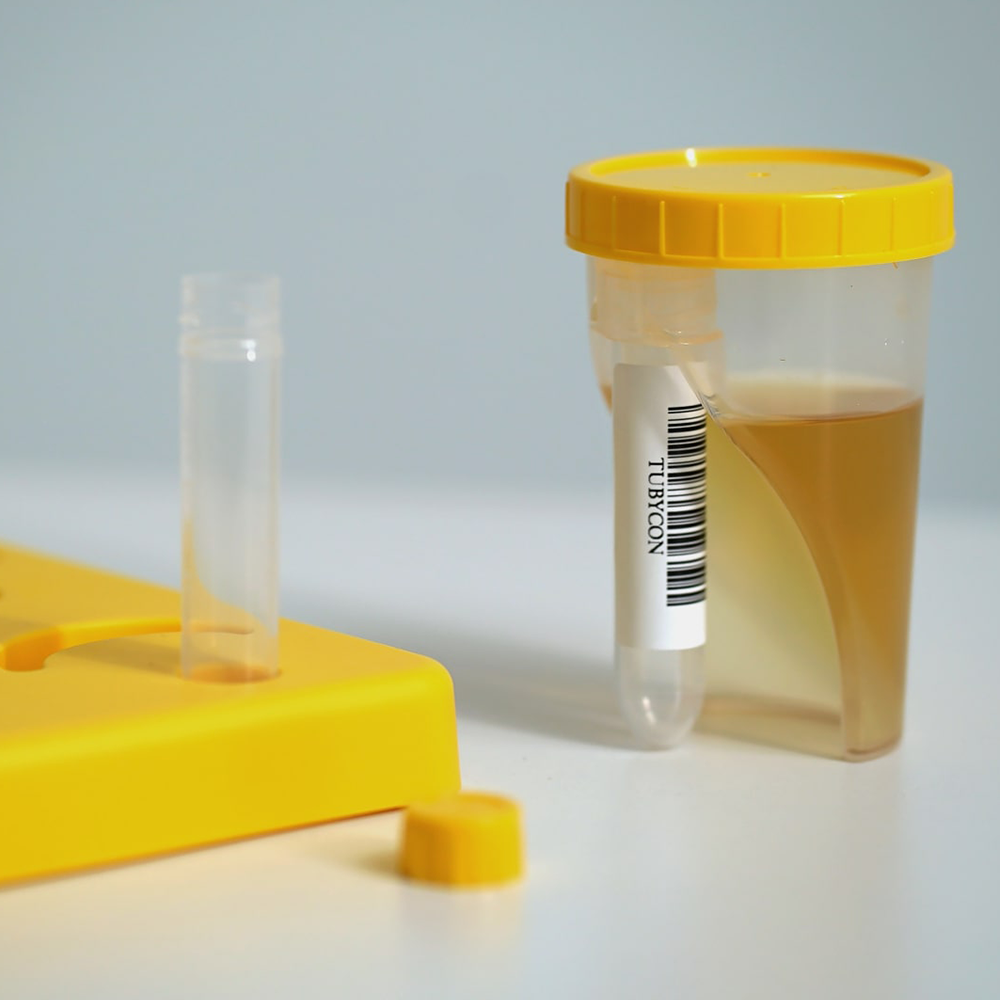
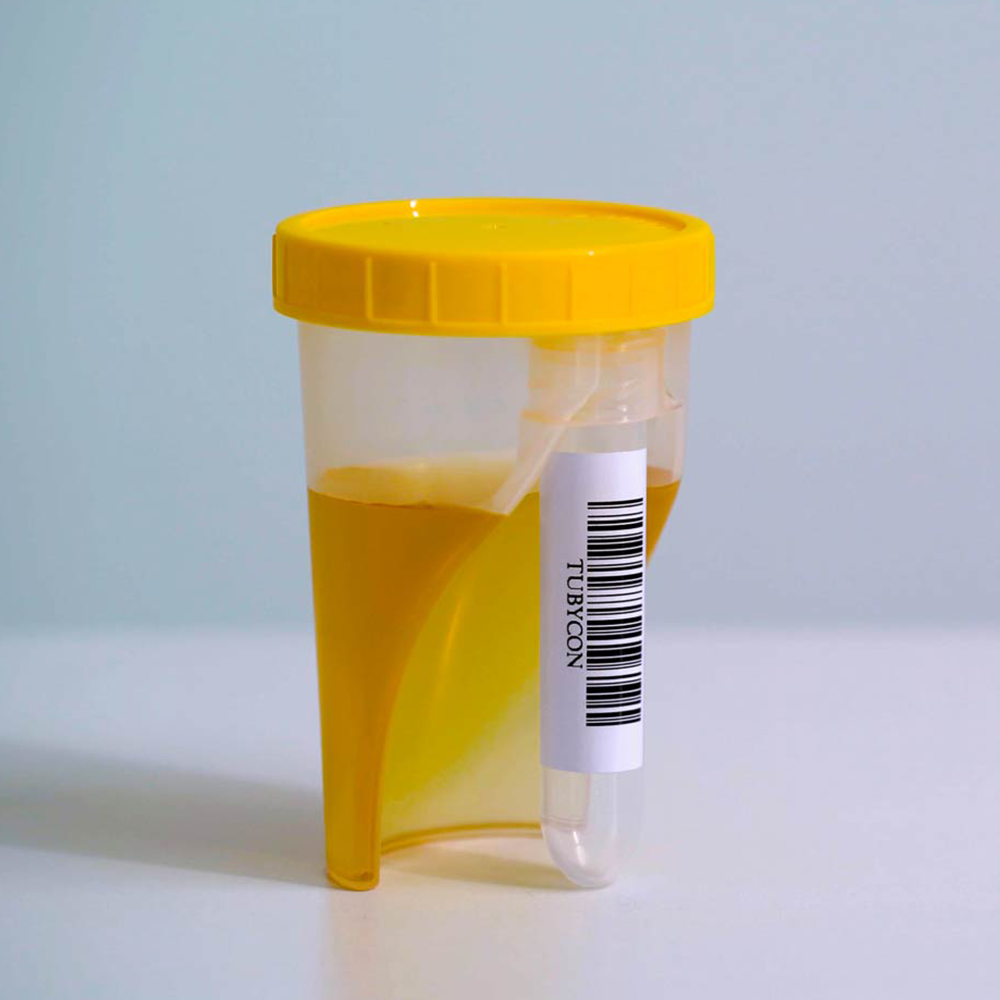
When the cup is tilted toward the tube, the collected urine automatically flows from the cup into the tube, reducing the risk of contamination and specimen mix-ups while streamlining the testing workflow.
· Main Functions
The cup and tube are combined into a single structure. The tube is detachable with a threaded design and comes pre-labeled with a barcode. Compatible with laboratory automation equipment and pneumatic tube systems.
Advantages
· Primary Advantages
No additional transfer steps are needed as the urine flows directly into the tube, improving work efficiency and eliminating contamination risk.
· Competitiveness
FDA-registered product manufactured in an ISO 13485 and GMP-certified facility.
How to Use
1. Check that the product is intact and not contaminated.
2. Collect urine directly into the cup.
3. Tilt the cup to transfer the desired amount of urine into the tube.
4. Detach the tube by turning it counterclockwise.
5. Dispose of the used cup and cap as medical waste.
2. Collect urine directly into the cup.
3. Tilt the cup to transfer the desired amount of urine into the tube.
4. Detach the tube by turning it counterclockwise.
5. Dispose of the used cup and cap as medical waste.
Cautions
· Single-use only – do not reuse.
· Do not reattach the tube after removal.
· Store at 4–25°C, away from direct sunlight and high humidity.
· Always check for damage or contamination before use.
· Do not reattach the tube after removal.
· Store at 4–25°C, away from direct sunlight and high humidity.
· Always check for damage or contamination before use.
Certifications
· ISO 13485 (OEM factory)
· GMP (OEM factory)
· CE IVDR Class A (in progress)
· FDA Registered
· GMP (OEM factory)
· CE IVDR Class A (in progress)
· FDA Registered
Awards
· Bronze or higher in the 2024 Good Design (GD) Award by the Ministry of Trade, Industry and Energy
· 1st Prize in 2024 IR Funding Competition (KRW 70 million) by Gwangju Technopark
· Selected for Korea’s NEP (New Excellent Product) Certification in 2025
· 2nd Prize at The New York K-Startup Pitch Competition 2022
· Commissioner’s Award from the Korean Intellectual Property Office at the 2022 Challenge! K-Startup Finals (Top 10 out of 5,400 companies)
· Commissioner’s Award from the Korean Intellectual Property Office at the 2022 IP Startup Competition
· Invention Culture Award by Nam Jonghyun Foundation in 2022
· Winner of 2022 Good Design (GD) Award by the Ministry of Trade, Industry and Energy
· Gold Prize at the 2022 Geneva International Exhibition of Inventions (Switzerland)
· Gold Prize at the 2021 Seoul International Invention Fair
· Presidential Commendation at the 2021 Korea Excellent Trademark & Design Fair
· Selected as an Excellent IP Startup by the Korean Intellectual Property Office in 2022
· 1st Prize in 2024 IR Funding Competition (KRW 70 million) by Gwangju Technopark
· Selected for Korea’s NEP (New Excellent Product) Certification in 2025
· 2nd Prize at The New York K-Startup Pitch Competition 2022
· Commissioner’s Award from the Korean Intellectual Property Office at the 2022 Challenge! K-Startup Finals (Top 10 out of 5,400 companies)
· Commissioner’s Award from the Korean Intellectual Property Office at the 2022 IP Startup Competition
· Invention Culture Award by Nam Jonghyun Foundation in 2022
· Winner of 2022 Good Design (GD) Award by the Ministry of Trade, Industry and Energy
· Gold Prize at the 2022 Geneva International Exhibition of Inventions (Switzerland)
· Gold Prize at the 2021 Seoul International Invention Fair
· Presidential Commendation at the 2021 Korea Excellent Trademark & Design Fair
· Selected as an Excellent IP Startup by the Korean Intellectual Property Office in 2022
Basic Product Information
| Product Name | TUBYCON-U Urine Sample Collection Single Kit |
| Brand Name | TUBYCON |
| Weight | Approx. 26.2g |
| Height | 97mm |
| Width | 66mm |
| Expiration Date | 5 years |
| Origin | Republic of Korea |
Warranty Period
Not applicable (single-use medical device)
Warranty Description
This product is designed for single use. No warranty applies if reused, and reuse is prohibited.
Customer Reviews
“We can now collect samples accurately and without leakage, significantly speeding up the test process.”
– National University Hospital, Korea
“Safer and more convenient than conventional cups.”
– Korean Public Medical Institution
– National University Hospital, Korea
“Safer and more convenient than conventional cups.”
– Korean Public Medical Institution
Frequently Asked Questions (FAQ)
About Us
Company Overview
TUBYCON is a specialized manufacturer of urine collection containers, founded by a clinical laboratory professional with field experience in hospital settings. The company develops products with a focus on infection prevention, work efficiency, and patient safety. TUBYCON holds multiple intellectual property rights and global certifications.
Factory Information
· Location: Hwasun, Jeollanam-do, Korea
· Production Partner: DXM (ISO 13485 and GMP certified)
· Production Capacity: Over 2 million units per month
· Production Partner: DXM (ISO 13485 and GMP certified)
· Production Capacity: Over 2 million units per month
Our Service
· OEM/ODM custom production
· Buyer logo insertion and packaging customization
· Support for export x-documentation and certifications
· Buyer logo insertion and packaging customization
· Support for export x-documentation and certifications
Our Policy
· Quality-first manufacturing principles
· Compliance with international standards
· 24-hour customer response system
· Compliance with international standards
· 24-hour customer response system
R&D CERTIFICATE
PAYMENTS DETAILS
This payment are available by Gobiz.com system.
- P@yGOS(PayPal)
- Telegraphic Transfer : T/T
- Name : Jehyun Park
SHIPPING
Shipping from :
Republic of Korea
- 150 Donggyecheon-ro, Dong-gu, Gwangju (61436)
TUBYCON
The person in charge
JEHYUN PARKAddress
150 Donggyecheon-ro, Dong-gu, Gwangju (61436)
튜비콘 제품
TUBYCON
Introduction
TUBYCON prioritizes patient safety and healthcare professional convenience, focusing on creating reliable hospital environments. Our flagship product, the all-in-one urine cup ‘TUBYCON-U’, prevents infections and medical errors caused by specimen mix-ups while maximizing work efficiency.
Through continuous research and development, we have secured over 77 intellectual property rights across 56 countries worldwide, establishing ourselves as a global leader. With innovative technologies and solutions, we provide safety and convenience to both patients and healthcare professionals.
-
- Business Type :
- Manufacturer
-
- Main Product :
- URINE SPECIMEN CONTAINER, URINE CUP, URINE SAMPLE CONTAINER, TUBYCON-U, TUBYCON
-
- Established :
- 2022-03-28
-
- Total Annual Revenue :
-
- Total Employees :
- Less than 5
R&D CERTIFICATE
-
- FDA Class I
- FDA
- 20231023
- 인증서보기
-
- Biocompatibility Test
- KTL
- 20231227
-
- IP67 Water and Dust Resistance Testing
- KTL
- 20240618
-
- FDA
- U.S. FOOD & DRUG
- 2023.10.23
- 인증서보기
-
- Test Report IP67
- ktl
- 2024.06.18
- 인증서보기
-
- Biological safety testing final Report
- ktl
- 2023.12.26
- 인증서보기
-
- Biological safety testing
- ktl
- 2023.12.19
- 인증서보기
-
- Biological safety testing
- ktl
- 2023.12.26
- 인증서보기
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
-
- Total export revenue (previous year in USD)
-
- Number of foreign trade employees
- Less than 5
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
-
 GOLD
GOLD
- TUBYCON Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★